One of the most recognised faces of the last century is that of Albert Einstein. Very many people know a little about Einstein, the eccentric old genius with the fright-wig hairstyle and rumpled clothing. Many people are also aware of his great intelligence. In some parts of the English-speaking world, the word 'Einstein' has become a generic term referring to a very intelligent person.

People who know something about science are also aware of his
theories of relativity: the Special Theory of Relativity
(published in 1905) and the General Theory of Relativity (published
in 1915). What many people do not know is that Einstein was
the second person to make a major contribution to the quantum
revolution, in a paper also published in 1905 [1]. In fact, this paper won him a Nobel prize.
The paper in question was about the nature of light [2]. Albert Einstein's contribution to this
discussion was a theoretical explanation of a certain phenomenon,
which showed that light was a stream of particles (which would
later be named photons).
This phenomenon is called the photoelectric effect. When you shine a light upon certain metals, a stream of particles (later found to be electrons [3]) is emitted from that metal. The emission has been found to have certain properties.
- The number of electrons emitted by the metal depends on the intensity of the light beam applied on the metal; more intense the beam, higher the number of electrons emitted.
- The emitted electrons move with greater speed if the applied light has a higher frequency.
- No electron is emitted until the light has a threshold frequency, no matter how intense the light is.
If light were to be a wave, both the energy and the number of the electrons emitted from the metal should increase with an increase in the intensity of light. Observations contradicted this prediction; only the number, and not the energy, of the electrons increased with the increase of the intensity of the light.
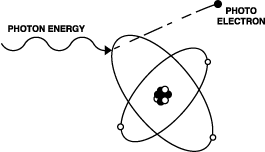
What Einstein showed was that the photoelectric effect as it had been observed could be explained if individual particles (or quanta) of light were penetrating the metal and knocking electrons loose from atoms. According to Einstein's paper, increasing the intensity of the light increased the number of photons, while the energy of each individual photon remained the same, as long as the frequency of the light remained the same. Therefore the number of electrons emitted would increase, but the energy transmitted to them by the particles of light would remain the same.
In one stroke Einstein showed that light is a stream of particles, and also that there was solid evidence for the existence of quanta. His theory could satisfactorily explain all the known properties of the photoelectric effect, and was the first result derived from quantum theory of the interaction between radiation and matter.
The same theory also raises a fundamental question: is light ultimately a wave, or a stream of photons?
The answer is: both. Light behaves as a wave under certain conditions, and as a stream of particles under others. It is said to have a dual nature: we can understand it as either wave or particle, depending on our context of observation. If you are wondering why this is so, rest assured that this matter will be clarified later, in Chapter 7.
Footnotes
1. Einstein's paper was titled, in German, Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt or, in English, On a Heuristic Viewpoint Concerning the Production and Transformation of Light. Back2. Normally when we use the word light, we are actually referring to visible light. Visible light, however, is only a small part of the electromagnetic spectrum, which ranges all the way from radio waves to gamma radiation. Every kind of radiation on the electromagnetic spectrum is really the same thing, only with different wavelengths and frequencies. Back
3. Electrons will be explained in the context of atomic structure in the next chapter. Back
* Fig 3-1 Courtesy: Berkeley.edu